Search result for Clover Biopharmaceuticals
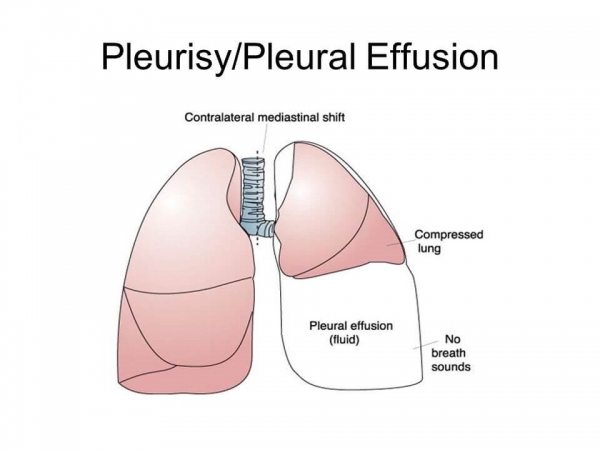
Phase 1 SCB-313 for Malignant Pleural Effusion Treatment tested by Clover Biopharmaceuticals in Australia
According to an announcement by an international biotechnology company in China, Clover Biopharmaceuticals, thriving to develop new biological therapies, the first-ever cancer patient suffering from malignant pleural effusions was admitted in Australia in a fully human TRAIL-Trimer fusion protein...
Australia: Clover Biopharmaceuticals tests Phase 1 study of SCB-313 for Malignant Pleural Effusion
CHENGDU, China ( November 21, 2019 ) Clover Biopharmaceuticals, an international clinical-stage biotechnology company based on the development of novel and revelatory biological therapies, announced today that the very first patient was administered in Australia for the treatment of cancer patien...

First Patient Dosed in Phase I Study of SCB-313 for MPE by Clover Biopharmaceuticals
International clinical-stage biotechnology engaged in generating new and transformative biologic treatments, Clover Biopharmaceuticals publicized today how they had dosed their first patient in a Phase I trial of SCB-313 in Australia for treating cancer patients having malignant pleural effusions...
